Rock Groups
If you’ve seen the Rock Hall of Fame, you may have noticed that the most common minerals in the earth’s crust aren’t single minerals that stand alone, like fluorite or amethyst, but groups of minerals that have general names that cover all the minerals in each group.
If you’ve read the Rock Science page, you may remember that a scientist named Dana created “classes” to show how certain minerals were made of similar elements. Those classes aren’t the same as these rock groups. These most common mineral groups are one of two things that are kind of opposite: 1) minerals that were different enough that they had separate names for a long time, but then someone realized they were all just different versions of the same basic thing, 2) minerals that were thought to be unique, but then it turned out you could find different variations of them with slightly different amounts of elements in them.
You wouldn’t be wrong if you thought those two things are really just two ways to look at the same situation. Either way, people ended up coming to the same conclusion: these are rock groups. And every good rock group needs a catchy name. Of course, what’s catchy to a mineralogist…might not always go viral in the rest of the world.
The following are some of the “most common minerals” in the earth’s crust or, more correctly, the most common rock groups. More groups are coming soon!
Feldspar
As the most common mineral in the earth’s crust, feldspar could fill an entire book with its varieties, chemistry, crystal forms, and localities, but here we’ll just provide a summary of the basics of what’s going on in the world of feldspar.
The Feldspar Endmembers
As noted in the Rock Gallery and the Hall of Fame, many minerals on this site and out in the world are actually varieties of feldspar: labradorite, moonstone, sunstone, etc. However, when scientists talk about feldspar, they consider the most important way to divide up the types of feldspar is by how much potassium, sodium, and/or calcium they have in them. They’ll refer to feldspar minerals called “endmembers,” which are the ones that are at the far end of the spectrum because they have the most potassium, sodium, or calcium in them.
The feldspar with the most potassium is called “K-feldspar,” which is an oddly dull name, but it refers to the element potassium, whose symbol in the periodic table of the elements is “K.” You can see that in the K-feldspar formula: KAlSi3O8 (potassium, aluminum, silicon, and oxygen). Note that the Si3O8 part means it’s a silicate mineral, like all other feldspars.
The feldspar with the most sodium in it has a much more interesting name: albite. Its name comes from the Latin word for “white,” since that’s its standard color. Its formula is NaAlSi3O8, which you might notice is not very different from K-feldspar, except it has sodium instead of potassium.
The calcium “endmember” also has a pretty interesting name: anorthite. Instead of color, its name refers to its shape, coming from the Greek and meaning “not right angle,” for its lack of right angles in its crystals. The formula for anorthite diverges a little bit from the other endmembers but is pretty recognizable as a feldspar: Ca(Al2Si2O8).
K-feldspar is pretty common, and albite is pretty common, but anorthite is pretty rare, so you won’t hear about it a lot when rockhounds are talking about feldspar.
Other Major Varieties
Still with me? Along with the fun names of The Rock Readers minerals mentioned above, and the three endmembers, when scientists talk about feldspar, you’ll hear a lot about “the -clases”—plagioclase, oligoclase, orthoclase, anorthoclase—along with microcline, andesine, sanidine, bytownite, etc. (-Clase rhymes with “ace” by the way, like in a deck of cards.) All of those minerals are kind of like mile markers in the feldspar continuum, with the different names matching how much potassium, sodium, or calcium—or a combination of them—they contain. Scientists have put together a pretty helpful triangular chart that shows where the minerals are on the continuum. A scientist named Alex Strekeisen (aka Alessandro Da Mommio) created (or at least provided) a very nice version of the chart, which is shown here.
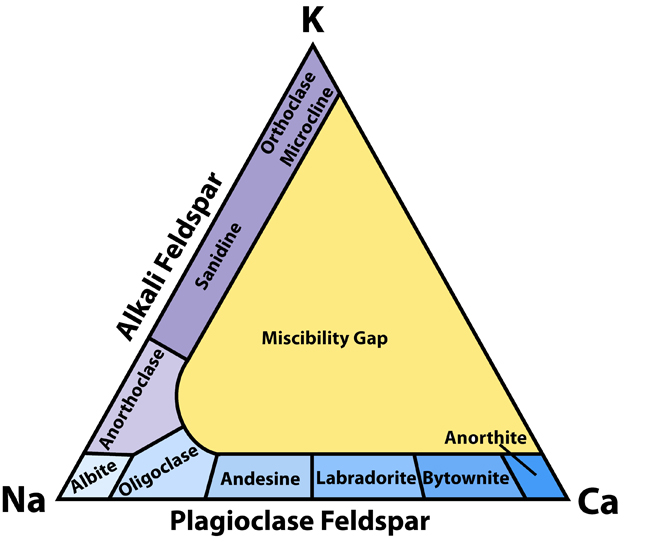
If you look at the chart, you can see that the feldspars can be broken down in yet another way into alkali feldspar and plagioclase feldspar. Man, feldspar is complicated! More specifically, though, the chart shows that feldspars can have all sodium, more sodium than potassium, more potassium than sodium, or all potassium—that’s along the left side. It also shows that they can have all sodium, more sodium than calcium, more calcium than sodium, or all calcium—that’s along the bottom. I guess potassium and calcium don’t get along very well though, because between them, you have to be either all potassium or all calcium!
Regarding microcline, mentioned above, like anorthosite, its name refers to the angles in its crystals, which have “small inclines,” so to speak, per the Greek meaning. The reason it’s worth mentioning is that it accounts for some pretty nice collectable crystals. Sometimes when you’re out rockhounding in a pegmatite, if you’re not lucky finding gemstones, you might find a nice microcline or two, which are usually white and kind of blocky but have a distinctive angled face in their termination. Along with the standard version, however, there’s this variety of microcline you just may have heard of, or seen in The Rock Readers Rock Gallery: amazonite. Amazonite is the most famous variety of microcline, and maybe even of feldspar, among rock collectors, with its attractive blue-green color.
Finding Feldspar in the Field
Lastly, a hint about collecting feldspar in the field. One common problem nearly every rockhound runs into, unless they have godlike mineral ID powers, is how to tell what a mineral is when it’s all dirty and in a pile with a bunch of rocks that are all dirt-colored. In that case, shape can help, but the most common feldspar has a special feature that can make it pretty easy to identify.
If you have a chunk of off-white, drab green, slightly pinkish, slightly orangish, or slightly brownish rock, given feldspar’s prevalence, there’s a good chance you have a piece of feldspar. But the clincher happens if you can clean off one side of it and hold it in the sun, turning it slightly back and forth in the light. If it’s feldspar, at one point the whole surface of that side will catch the light and flash at you—I call this, not surprisingly, the feldspar flash.
Because of its internal crystal structure, not only will the feldspar flash, but within the flash you may see what looks like a network of subcrystals that make up the main flash. If you’ve ever seen a piece of elestial quartz, you’ve probably seen how it often has a lot of little crystal terminations on the piece that will all flash at you if you turn it the right way. The feldspar flash is kind of like that, except it’s from the internal crystal structure, not from having a lot of external crystal terminations.
Hopefully the feldspar flash method will help you find all sorts of feldspar specimens…and then you’ll have to figure out which feldspars they are—good luck!
Sources for This Article (in addition to our* personal knowledge):
Alex Strekeisen, Feldspar: https://www.alexstrekeisen.it/english/vulc/feldsparstructure.php
*Note from Amy: Carl is being kind. We all know it’s HIS personal knowledge, right?
Quartz
Quartz is the second most common mineral in the earth’s crust after feldspar, and fortunately with second place comes a much less complicated chemistry. While feldspar mixes in sodium, calcium, and/or potassium and gets called all sorts of different names depending on how much of any of those it includes, quartz is relatively sitting pretty with its simple silicon dioxide (SiO2) formula with maybe a touch of this or that added.
Of course, if you’ve been exploring The Rock Readers website, you’ve probably noticed that quartz varieties have plenty of different names too. But in this case the names are related to what the mineral looks like or how it formed, as opposed to what its formula is.
The Big Crystal Division
The biggest division in the world of quartz is between crystalline and cryptocrystalline. It’s not exactly an even split, but it is a split that makes a lot of sense when you look at all the different minerals in the quartz group and see how different the two types look.

Crystalline quartz is, not surprisingly, quartz that forms obvious crystals. Another way to describe such crystals is to say they are “euhedral,” which comes from the Greek for “well-formed surfaces,” so they are crystals with well-formed surfaces.

Cryptocrystalline quartz, on the other hand, is quartz that forms very nonobvious crystals. In fact, the prefix “crypto-” means “hidden.” The reason why cryptocrystalline quartz’s crystals are considered “hidden” is that they’re actually tiny little grains that are so small that when you look at a mineral that’s made up of them, its surface looks smooth. You’d have to zoom in closely with a microscope to see the crystals that are there, and you’d see that they’re all packed together, with no room for making pretty “euhedral” shapes.
The Minerals and Rocks in Each Category
For euhedral quartz, all of the varieties are considered minerals. For cryptocrystalline quartz, some varieties are considered minerals, and some are considered rocks, and some people think they should all be considered rocks because of the other minerals that are mixed in. We won’t get into that argument. What we will do is tell you the names of the varieties and a little bit about them. Not too much, though, because you can get a lot more information about many of them by finding them elsewhere on this website! (We’ll provide links.)
Euhedral Varieties
At the top of the group of euhedral quartz varieties sits what you might call the mother of all quartz, which we at The Rock Readers refer to by the fairly simple name “clear quartz.” However, one of the older, more classic names is “rock crystal,” and you’d be correct if you assumed people used that name in the old days because it was so universally considered the definitive crystal. Some people still call it rock crystal to this day.
Group Leader: Clear Quartz—Colorless transparent quartz
Varieties:
- Amethyst—Purple translucent quartz
- Ametrine—Quartz that’s part amethyst and part citrine in the same crystal
- Blue Quartz—Blue translucent to opaque quartz, which is pretty uncommon
- Citrine—Yellow to orange translucent quartz
- Milky Quartz—White opaque quartz, which is the most common
- Rose Quartz—Pink translucent to opaque quartz
- Smoky Quartz—Black or gray translucent to opaque quartz
Cryptocrystalline Varieties
As with euhedral quartz, cryptocrystalline quartz has its figurehead, and that would be chalcedony. Some people consider chalcedony to be just a type of crypto quartz, while others consider it to be the only type, with all other types actually being subtypes of chalcedony. On The Rock Readers website, we refer to several minerals as types of chalcedony, as noted in the list below.
Group Leader: Chalcedony—Crypto quartz that comes in several different colors
Varieties:
- Agate—Chalcedony with bands/layers, and other formations, that range from translucent to opaque and have different colors and tones
- Carnelian—Red to orange translucent to opaque chalcedony
- Chert—Opaque crypto quartz of various colors that forms in sedimentary rocks
- Chrysoprase—Milky green opaque chalcedony, with nickel causing the green color
- Flint—A type of hard chert that forms in chalk and other sedimentary rocks
- Jasper—Opaque reddish, yellowish, greenish, or multicolored chalcedony with a large amount of an included mineral or minerals
- Onyx—Black and white opaque chalcedony
- Tiger’s Eye—Brown and gold crypto quartz with a chatoyant effect
How the Two Types Form
If you spend much time in the world of minerals and hear a bit about the science, you get to know that some of the science hasn’t been nailed down as well as, say, chemistry. One of the biggest problems is that rocks and minerals tend to form on a geologic time scale, which is not a very human time scale. If you’re a chemist, you can combine two chemicals, and BOOM! you’ve just learned not to combine those two chemicals. In geology and mineralogy, unless you have extremely fancy equipment, you might have to sit around for a million years to wait for something to happen, and most people don’t have that kind of time.
So, for example, there are different theories out there regarding how long it takes for pegmatites to form, how geodes form, and how cryptocrystalline quartz forms. However, there is general agreement that crystalline and cryptocrystalline quartz form under different conditions, and people have some pretty good ideas for what those conditions might be, so we’ll talk a little about what seems reliable at this point.
According to Amir C. Akhavan’s The Quartz Page (http://www.quartzpage.de/index.html), two conditions seem to have a lot of influence on whether quartz forms in a “crypto” or “euhedral” way: the temperature of the environment where the mineral is forming and how much silicon is available there. If the temperature is above about 150°C (302°F) and there’s only a little bit of silicon available “in watery solutions,” euhedral quartz is more likely to form. If the temperature is lower than that and there’s a lot of silicon available, crypto quartz is more likely to form.
What does Amir mean by “watery solutions”? Quartz often forms from hydrothermal fluids, which generally means water that’s heated deep underground; dissolves minerals in the area and along the way to wherever it’s headed; and often moves upward because of heat, pressure, and/or actual magma pushing it upward. The “watery solution” is this water with dissolved minerals in it.
Euhedral Formation
Why would euhedral quartz form at high temperatures when there’s some but not much silicon around? Well, every mineral—okay, let’s say “most” minerals—has a particular way it likes to crystallize. That’s what we talk about in the Rock Science page when we talk about a mineral’s “crystal system” (shape). A mineral with a cubic crystal system really wants to form in a cube shape if at all possible. For quartz, which has a hexagonal crystal system, it really wants to form a hexagon shape.
To form perfect shapes, at least some minerals prefer to form slowly and carefully enough that the building blocks have time to stack up in the correct order. For quartz to form its perfect shape, it needs to avoid being mobbed by too many building blocks at once, and that’s why having some but not a lot of silicon available is a good thing. Once a crystal starts to form, the silicon atoms and oxygen atoms can combine into quartz building blocks gradually and be attracted to the correct empty spots in the crystal structure. If they come too quickly, the shape won’t be perfect—it may even be downright weird! Having the water temperature higher is important just to keep it a liquid and keep the silicon that’s floating around in it from solidifying too quickly.
Crypto Formation
The science behind forming euhedral quartz crystals seems fairly well defined, whereas the science behind forming cryptocrystalline quartz is an area that’s still a bit fuzzy. Some people talk about silicon precipitating (solidifying in tiny grains or, as Peter Heaney suggests, forming “fibrous crystals”) out of water and those grains (or fibers) combining together into a solid, while others talk about silicon forming a jelly that hardens into a solid, or maybe it’s both! Whichever it is, as noted earlier, you need to have water/jelly that has a large amount of silicon in it—it should be pretty “saturated.”
Sometimes the fluid involved can be pretty simple and be made up of nearly all water and silicon with very little other minerals. In that case, the cryptocrystalline quartz might solidify as translucent or transparent and very light colored or colorless. Or the fluid might have large amounts of other minerals, such as iron or manganese, which can solidify with the quartz to make it, for example, reddish or purplish, respectively, in which case the quartz is likely to have a more intense color and be opaque.
How is it that things like geodes and agates have different-colored layers? Well, usually they form inside air bubbles or gaps in rock, and what happens is that silicon-saturated fluid gets in there, and the quartz solidifies in layers. That layering could happen from different mineral particles being caught in the quartz at different times from the same fluid as the silicon slowly precipitates out and hardens, or from a little liquid getting in there, drying out or draining out and leaving a layer of one color of quartz, a little more liquid going in and leaving another layer, and over and over, each time bringing in different amounts of different minerals.
Unfortunately, we haven’t been able to stick tiny, extremely long-lasting cameras into any of these bubbles/gaps to watch the process happen. But there’s pretty good evidence that the general description above is fairly accurate. What’s important to us in the end is that the whole process leads to a cryptocrystalline quartz formation that looks good enough to keep in our collection, or use as bookends, or make a nice polished cabochon, or….
Sources for This Article (in addition to our* personal knowledge):
Amir Akhavan, The Quartz Page: http://www.quartzpage.de/index.html
Nancy Marie Brown, How Do Agates Form?: https://www.psu.edu/news/research/story/how-do-agates-form
Geology Science, Chalcedony: https://geologyscience.com/minerals/silicates-minerals/chalcedony/#Formation_of_Chalcedony
*Carl’s!
Mica
After feldspar, quartz, and pyroxenes, and tied with amphiboles, mica is one of the most common (and well-known) minerals in the Earth’s crust. You’ve probably heard of it before, and if so, you probably think of thin, silvery sheets that you can even see through, which means you’re thinking specifically of the type of mica called muscovite. Muscovite is such a common and useful type of mica that most people think of the two things as one and the same. Ah, if only it were so simple!
According to a scientist named Robert Lauf, mica actually can be any of 40 or so different minerals that all form in stacks of sheets similar to (and including) what you see with muscovite. Fortunately for us, most of those 40 minerals are the kinds of minerals only interesting to professional mineralogists and collectors who just have to have one of everything. A number of the different micas can look very similar, so in many cases you have to have access to sophisticated and expensive testing equipment to tell what exact kind of mica they are.
For the rest of us, on any given day out rockhounding we’re likely to only come across a few different varieties of mica. What makes our lives both simpler and more complicated is that of these few types, much of the time we want to know what they are so we don’t bring them home with us. The simpler part is what we want to keep; the more complicated part is what we need to filter through and get rid of so we can find the minerals we really want.
The Most Common Micas
The two most common varieties of mica you’ll normally find are muscovite and biotite, probably the lightest and darkest micas, respectively. These usually fall into the “get rid of” category.
Muscovite
You know something (muscovite) is just a little too common when you leave a trail of it from the mine, into your car, and into your house or apartment, then find more silvery flakes in your laundry later. When you’re at a mine that’s in pegmatite rock, for example, you may literally have to sift through 15 pounds of muscovite to find a single small piece of aquamarine or tourmaline. So you spend a lot of time just moving mica out of the way.
Another time when muscovite gets in the way is when you’re gold panning. Even though it’s very lightweight and should be washed away early on in your panning session, a few flakes are always left toward the end. When you’re trying to figure out what the gold-colored piece is that you see, you end up having to waste a bunch of time fiddling around to determine if it’s actually gold or just an annoying golden muscovite flake.
On the other hand, when something is so common, there are pretty good odds that you might find a nice piece worth keeping! You can find sheets of muscovite up to six inches or more across; you can find thick stacks of perfectly hexagonal muscovite; and you can even find rounded, ball-shaped or “ball-peen” muscovite that looks similar to shiny botryoidal hematite crystals. These can all be quite attractive or impressive additions to your collection, although you should watch that you don’t get quickly buried in muscovite specimens. One exception with the hexagonal muscovite stacks is that when they’re thick but not wide stacks, they can look a lot like beryl or apatite crystals when they’re embedded in a rock, and it can be pretty disappointing when you realize you’re looking at mica, not a nice gemstone.
Muscovite is usually silver or slightly golden, although it can also be green (called “fuchsite,” which is pronounced “phewk-sight”), or pink, or other colors. Compared to other micas, muscovite has the most aluminum (Al) in it, with the formula KAl2(AlSi3O10)(F,OH)2.
Biotite
Biotite is the next most common mica that you’ll run into. If you’re out field collecting, it’s maybe one-tenth as common, but sometimes there will be quite a lot of it. Sometimes it’s the only kind of mica you’ll find in a particular place, but once in a while you’ll find locations where there will be muscovite and biotite in the same general area, although usually not mixed together in the same rock.
Fortunately, biotite is pretty easy to recognize. It usually forms these dark brown narrow rectangles made of the typical thin stack of sheets. Usually you’ll find them embedded in feldspar or quartz, but sometimes they’ve popped out and are loose on the ground. They can look kind of neat, and sometimes they form thick, heavy stacks that can be pretty impressive, so it can be worth keeping a piece or two as a souvenir of the locality.
How is that biotite is so much darker than muscovite? Where muscovite has all sorts of aluminum, biotite has a bunch of iron (Fe)…and a little aluminum (Al), with the formula K(Mg,Fe)3AlSi3O10(F,OH)2. It’s the iron that makes it such a dark color. Note that there’s a comma and an “Mg” before the Fe. That means it can contain different amounts of magnesium or iron, with the most magnesium-y biotite being called phlogopite, and the most iron-y biotite being called annite. Nowadays they call biotite a “series” rather than a mineral because it includes that range of minerals. But when you’re out collecting, all you’ll care about is that it’s biotite.
The Coolest Mica: Lepidolite
Ask anyone who’s been to a pegmatite mine, and they’ll tell you the coolest mica is lepidolite. Lepidolite really stands out because it has such a striking color, ranging from pinkish purple to a deep bluish purple. It can come in sheets like other micas, but the sheets tend to be much smaller than something like muscovite. On the other hand, it also forms in stacks as muscovite does but often in a much more impressive and interesting way.
Beyond muscovite-style stacking, for one thing, lepidolite can form solid shapes that look similar to stacks though they’re not made up of separate layers, but it also can go so far as to form in solid deposits big enough to be large lepidolite boulders! Some of these boulders have been carved into vases and statues—try that with muscovite!
As with biotite, lepidolite sadly no longer has the honor of being its own defined mineral, it’s now considered a “series” of minerals. Lepidolite includes different minerals that have different amounts of lithium and aluminum, but the difference is not quite as obvious as with biotite and its iron and/or magnesium. One end of the lepidolite spectrum is called polylithionite KLi2Al(Si4O10)(F,OH)2, whereas the other end is called trilithionite K(Li1.5Al1.5)(AlSi3O10)(F,OH)2. Note that they both have “lithion” in their names because they both contain lithium. Again, fortunately you don’t have to know which one you’ve found, because they both look cool, and no one else will know the difference either!
Those Other Micas
The types discussed above pretty much cover any micas you’ll come across or hear about in your day-to-day rockhounding life. If you’re lucky, though, you might end up somewhere where you can find pink margarite mica flakes, dark brown phlogopite mica crystals, or one of the 30 or so other micas. Whatever it might be, if it looks worth keeping, why not keep it? Someday you might learn that you found something better than you thought!
Sources for This Article (in addition to our* personal knowledge):
Jolyon Ralph, The Most Common Minerals on the Earth: https://www.mindat.org/a/common_minerals
Robert Lauf, “Collector’s Guide to the Mica Group,” Rocks & Minerals, Volume 81, May/June 2006, also available as a book at https://schifferbooks.com/products/coll-gde-to-the-mica-group
*<cough> Carl’s <cough>.
Amphiboles
After feldspar, quartz, and pyroxenes, and tied with mica, amphiboles (as a group) are one of the most common minerals in the earth’s crust. What the heck is an amphibole? That’s a very good question, and we have some answers. We’ll explain the basics you need to know about this mysterious group.
First of all, what does the word amphibole mean? Like a lot of other mineral names, this name came from the Greek, in this case the Greek word for ambiguous (basically, “uncertain”)—they look kind of similar, right? That meaning makes sense because it can be easy to confuse different amphibole minerals for each other.
It’s very likely that if you’re new to the mineral world, you’ve never heard the word amphibole. You have a lot of company! What minerals do most people know about? Gemstones, precious metals like gold and silver, salt—that sort of thing. We know about those things because either they’re beautiful or colorful or we use them directly every day. Although certain amphiboles are useful to certain people and industries, most of us wouldn’t normally have anything to do with a chunk of actinolite, for example. And most of the amphiboles aren’t particularly colorful, so they wouldn’t grab your attention, and you wouldn’t make a necklace out of them.
Now that doesn’t mean that you’ve never heard of any of the amphibole minerals at all. We do mention some of them here and there in The Rock Readers website, and there’s even one that’s fairly well known…but often not identified correctly. Yes, we’re talking about hornblende, an actual specific mineral whose name is used by a lot of people as a catch-all for “black crystals I can’t identify.” In other words, from the Mineralogy textbook, “the name hornblende is commonly used to refer to any black amphibole.”
There can be a lot of similarities within the group of amphibole minerals, and some of those similarities apply when you compare amphiboles with the even more common group called pyroxenes, which we mentioned earlier and cover separately. According to William Nesse via Wikipedia, though, amphiboles are different from pyroxenes in two important ways: amphiboles have more silicon and more water in them. On the other hand, he also says that if you take away the water and heat up an amphibole, it can turn into a pyroxene. So they’re not that far apart.
The reason that amphiboles are so common isn’t because they’re forming all sorts of pretty crystals all over the place, it’s because they’re part of a lot of the rock out there, including several different specific types of rock. Nesse says that amphiboles are likely to be a part of a specific type of “igneous rocks such as diorite, granodiorite, andesite, and dacite,” along with more commonly known rocks such as granite, which usually contains hornblende. They’re also part of some metamorphic rocks such as, not surprisingly given the name, amphibolite.
Along with hard rocks, amphibole minerals can take the form of soft fibers. As you may know, asbestos is one “mineral” that occurs as fibers instead of being “as hard as a rock.” What you may not know is that asbestos isn’t itself a mineral, it’s a form that minerals can occur in, which mineralogists call asbestiform. Several different minerals can be asbestiform at times, and that includes such amphibole minerals as “crocidolite…, tremolite, and actinolite.” Back before asbestos was outlawed because of its bad health effects, amphiboles accounted for some of the asbestos that was used when it was popular as an ingredient in insulation, brake pads, oven mitts, and other products that needed to resist heat.
Just to be complete, we should mention that you can actually find some nice crystal specimens of some of the amphibole minerals. We already mentioned that they can be “asbestiform,” and, if we look at The Rock Readers habit chart, that’s the same as “fibrous.” As actual crystals, though, amphiboles are often radial but can also have acicular, prismatic, and other habits.
For example, crocidolite can occur as radial sprays of crystals, but it can also occur as needles (acicular) inside of quartz crystals, just like rutile does in rutilated quartz. Actinolite can occur as futuristic-looking radial crystals that seem to shoot out from their matrix rock, or they can be like a jumbled haystack made of dark green needles that, yes, you can get stuck in your finger if you’re not careful. Hornblende is known for pretty simple radial crystals that some people say look like birds’ feet. And richterite can form blocky crystals similar to topaz or amazonite, or prismatic ones like aquamarine. So even though some people look down on amphiboles for some reason, some of them can be perfectly nice minerals to add to your collection.
To sum things up, here’s a list of the most well-known (to some) amphibole minerals:
- Actinolite
- Anthophyllite
- Crocidolite (aka Riebeckite)
- Cummingtonite
- Glaucophane
- Hornblende
- Richterite
- Tremolite
There you have it! That’s probably more than you’ll ever need to know about amphiboles, unless you decide you want to be a professional geologist, in which case this will provide a start on the amphibole section of your first college mineralogy class.
Sources for This Article (in addition to our personal knowledge):
Dexter Perkins, Mineralogy, https://opengeology.org/Mineralogy/6-igneous-rocks-and-silicate-minerals-v2/#647_Amphiboles
Jolyon Ralph, The Most Common Minerals on the Earth, https://www.mindat.org/a/common_minerals
Wikipedia, Amphibole, https://en.wikipedia.org/wiki/Amphibole
William D. Nesse, Introduction to Mineralogy, https://archive.org/details/introductiontomi0000ness_m9a6
Geology is the Way, Amphibole, https://geologyistheway.com/minerals/amphibole-group/